Occasionally you may come across a molecule with an odd (as opposed to even) number of electrons, such as NO2.
This particular molecule has 17 electrons.
After counting electrons,
arranging the atoms, single bonding everything, you will have the
following LDS which is still short 13 electrons – the equivalent of 6.5
pairs, or 6 pairs and one single dot.
These are placed (as before) from the outside in, in pairs, giving us the LDS below which is still missing one electron.
That electron is added to the central atom (since the outside atoms already have octets).
However, the nitrogen doesn't yet
have an octet. To solve this problem we can try to move electrons into
bonds (as we have done before). This leaves is with two
possibilities—one where the nitrogen only has 7 electrons, and one where
the nitrogen has 9 electrons.
For various reasons that are beyond
the scope of this text, if we cannot achieve an octet, we fail on the
low side (7) rather than on the high side (9). In other words we choose
the first LDS above, rather than the second.
However, this still isn't quite right. We need to consider one more thing.
Resonance



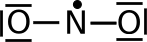


No comments:
Post a Comment