The technology of lithium-ion batteries is a huge area of research and, although what follows is only a surface explanation of what is happening, it may already be out of date by the time you read this.
A lithium-ion cell contains a positive end and a negative end which are connected by an electrolyte mixture that allows lithium ions to move from one end to the other through a separator that does not allow the flow of electrons.
The positive end contains one of several compounds such as \(LiCoO_2\). Because this is an ionic compound and because lithium ions are very soluble, the connection between the \(CoO_2^{-1}\) and the \(Li^{+1}\) ion is loose and tentative.
The negative end of the cell has a matrix that can hold lithium ions. This matrix is often made of graphite.
An uncharged (completely used up) lithium-ion cell looks like this:
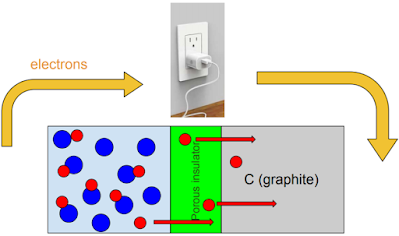
Charging a lithium-ion battery involves connecting the battery to a power source that can create a large negative charge on the graphite end of the battery and a positive charge on the Co end. This is done by moving electrons through the charger from the Co end to the graphite end.
As a result of this, the \(Li^{+1}\) ions are attracted to the graphite end. They move through the separator and lodge in the graphite matrix.
The result of this process is a charged battery. The power is stored in the potential energy found between the \(Li^{+1}\) ions and the \(CoO_2^{-1}\) ions.





No comments:
Post a Comment