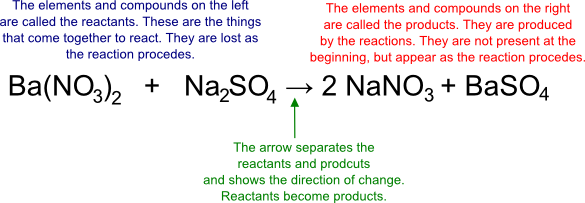
Here is the same reaction, with explanations for all of the numbers that you may or may not recognize:
When reactions are written in this manner, there are a few very important rules that must be followed:
- The reactants (the things that are reacting together) are always on the left side
- An arrow leads from the left to the right and signifies that a change is occurring.
- The products (the things that are formed in the reaction are always on the right side
- The reaction must be balanced. That means that there must be the same number of atoms of each element on both sides of the reaction.
- Formulas MUST be written correctly according to the defined practice
- Reactions are balanced by adding coefficients (by changing how many or each molecule are involved in the reaction not by changing the formula itself



No comments:
Post a Comment