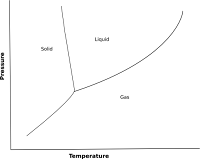
The solid/liquid line is nearly vertical on a phase diagram. However, nearly vertical means not actually vertical. As such, the line can either lean to the right or to the left.
Drawn in a very exaggerated way then the graph can either look like this
or like this
Why does this matter? Look at what happens when a vertical line is placed on the two graphs.
Starting at the bottom of the line and going up on the first graph would change a gas into a liquid and then into a solid. On the second graph the gas would turn into a solid first and then a liquid.
Why would different materials behave so differently? Remember that increasing pressure forces the particles closer together, so that the material goes into a denser phase (Pressure and phase changes). The difference between the two graphs depends on the densities of the various phases of the materials.
For substances that have a phase diagram like the one of the left, the density is highest in the solid phase, so increasing pressure will eventually force the material into a solid.
For substances that have a phase diagram like the one on the right, the liquid has a higher density than the solid, so tat increasing pressure will force the material into the liquid phase.
So...what kind of substance does that?
Welcome to aBetterChemText
Why aBetterChemText?
What is aBetterChemtext? aBetterChemText is intended to be a new way to look at Chemistry. It is written in plain English to make it acc...
Subscribe to:
Post Comments (Atom)
-
The Beginnings of Science and Chemistry This thread of history takes us through Ancient Greece , through Northern Africa during what is cal...
-
There are many real-world applications of the laws discussed in this section. They include: How you use a straw sucking in your cheek...
-
In the discussion of Planck’s work, I suggested that we could understand quantization as being like money - everything is a multiple of the ...




No comments:
Post a Comment