Lewis took the Octet Rule and
applied it to molecules. For instance, we already know that a chlorine
atom is unstable because it has only 7 electrons, and that it can become
stable if it can steal an electron from another atom to become an -1
ion. We also know that chlorine appears in nature as Cl2 not as single atoms of chlorine and that the molecule has a single bond.
Lewis deduced that if electrons
involved in the tug-o-war each counted for both atoms, then each atom
would have an octet and would be stable.
In other words, the chlorine atom on the left "owns" 7 valence electrons and is pulling on another, for a total of 8. The chlorine on the right has the same situation. As a result, each atom "feels" like it has 8 electrons - an octet.
Using this logic, we can draw the LDS's for other molecules, like OCl2:
Notice that the oxygen started with 6 valence electrons, so it needed to make 2 different bonds in order to "have" 8 valence electrons.
What all of this means is that every "single" electron that an atom has wants to find a "partner" through bonding. So nitrogen, with 5 valence electrons (1 pair and three singles) tends to make 3 bonds, while carbon, with 4 valence electrons, tends to make 4 bonds.
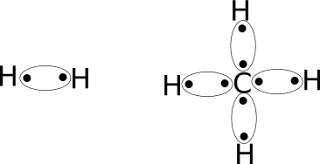
Lewis also determined that hydrogen
does not obey the octet rule, but rather follows its own “duet rule.” Of
course this makes sense when we remember that hydrogen only has one
orbital (the 1s) which can only hold two electrons. So LDS's that
involve hydrogen (H2, CH4) look like this:
Remember that Lewis didn't care how
the electron dots were placed around the atomic symbol. That means that
all three of the following are correct LDS's for water:
The last of these is a bit
disorganized but it shows, correctly, that water has one oxygen bonded
to two different hydrogen atoms.
For the sake of clarity we will, in the
future, arrange the electrons so that our diagrams are as neat as
possible.
It is also acceptable to replace any
pair of electrons (whether in a bond or attached to a single atom) with a
dash (—), as shown here:






No comments:
Post a Comment