The only way to make sense out of the jumbled mess of orbitals that Schrödinger’s theory suggests is to separate those orbitals by energy. Doing that allows us to “see” the orbitals separately, and that allows us to predict which orbitals will be occupied by electrons, and which will be empty in a given atom.
When scientists look at the energy of each of the orbitals a few things become apparent:
- Orbitals on lower energy levels have less energy than their exact counterparts on higher energy levels (this should not be shocking)
- The energy gap between energy levels is smaller between higher energy levels than it is between lower energy levels.
- Not all orbitals on the same energy level have the same energy (this is a little surprising!)
- The combination of numbers 2&3 means that there are some orbitals on higher energy levels that are lower in energy than some orbitals on higher energy levels. We’ll pull apart this idea below.
Let's take those ideas one at a time, starting with the first.
Orbitals on lower energy levels have less energy than
their exact counterparts on higher energy levels
In simplest terms, this simply means that the 1s is lower in energy than the 2s orbital is. So, if I were to make a "graph" of the s orbitals on levels 1-4 showing E as vertical height, it would look something like this:
However, this is NOT correct, because #2 makes things look very different.
The energy gap between energy levels is smaller between higher energy levels
than it is between lower energy levels.
The actual energy differences between the different levels is related to the square of the energy level. So level 2 is only 1/4th as far down (from the top) as level 1, and level 3 is only 1/9th, etc.
That leaves our graph of the s orbitals looking like this
The third idea requires us to focus on one energy level at a time.
Not all orbitals on the same energy level have the same energy
Let's look at level 3. This level has a single 3s orbital, 3 different p orbitals and 5 different d orbitals. The 3s orbital is the lowest in energy. The p orbitals are all slightly higher in energy than the s orbital. The d orbitals are a little higher than that.
So, just for level 3, our orbital energy graph would look like this
Note: the fact that the p and d orbitals have been drawn to the right has NO MEANING. It is just common practice to keep the diagram legible.
The fourth idea is where things get a little weird
The combination of numbers 2&3 means that there are some orbitals on higher energy levels
that are lower in energy than some orbitals on higher energy levels.
If you look at the diagram that showed the s orbitals to scale, you see that the 3s and 4s are fairly close in energy. If you then look at the the diagram of just the level three orbitals, you see that the 3d's are higher than the 3s.
It turns out, that the 4s is closer to the 3s, than the 3d orbitals are. In other words, the 4s is LOWER in energy than the 3d's are. Putting all of this together gives us a diagram like this:
Of course, the real goal of understanding all of this is to see where the electrons in an atom can be found. Let's explore that now.



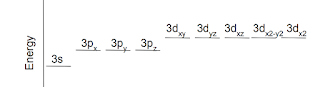


No comments:
Post a Comment