Finding the concentration of a solution
Imagine that you have a solution whose concentration was unknown. How would you determine the concentration? There are several ways to approach that problem. The most common are these:
Beer’s Law
Gravimetric Analysis
Titration
Beer’s Law
Beer’s Law (also called the Beer/Lambert Law) is used with solutions that absorb particular wavelengths of light - that is to say, solutions that are colored. When a solution is perfectly colorless, it is allowing all (or nearly all) wavelengths of visible light to pass through. When a solution has a distinct color, it is absorbing some of the visible wavelengths. For instance, a solution that looks green, is absorbing red light (the opposite color on the color wheel).
Beer’s Law says, in its simplest version, that the amount of light a solution absorbs is proportional to the concentration. In English, that means that a more concentrated solution looks darker. The details (which we will not delve into here) involve how we measure the amount of light absorbed, the amount of solution we are passing the light through and the nature of the solution itself.
Let’s take a simple example. If you dissolved a pack of Cherry Kool-Aid into some water the solution would be red. If you dissolved the packet into a cup of water, it would be a deeper, darker red than if you dissolved the packet into 2 quarts of water (as the directions state). If you dissolved that packet of Kool-Aid in a bathtub of water, the red color would be very pale indeed.
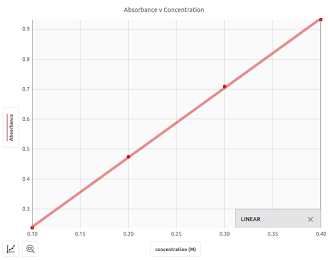
Using a device called a colorimeter, or spectrophotometer, we can measure the amount of light absorbed by the solution (using a unit called Absorbance). If we graph absorbance of the solution v. concentration (in molarity) we obtain a straight line graph.
If we then measure the absorbance of a solution whose concentration we do not know, we can use that linear graph to determine the concentration.
Gravimetric Analysis
Gravimetric analysis is used when either the cation or anion of the solution is generally insoluble. For example, potassium phosphate is soluble, but most phosphates are not, or silver nitrate is soluble but most silver compounds are not.
In that case, you can add a precipitating ion to the solution, filter, wash and dry the precipitate produced and then determine the amount of the ion that was present.
For example, if you had a solution of potassium phosphate whose concentration was unknown, you could measure out a sample (say 50.0 mL) of the solution, then add in excess barium nitrate. Barium phosphate will precipitate immediately by the reaction below.
2 K3PO4 + 3 Ba(NO3)2 → 6 KNO3 + Ba3(PO4)2
The precipitate can be filtered out of the solution. Once washed and dried, the mass of barium phosphate can be converted to moles of potassium phosphate in the original sample using the math below.
Dividing moles of K3PO4 by the volume of sample (in this example 0.0500 L) would give molarity.
Titration
Titration involves carefully adding a reactant to a measured sample until the reaction is just finished, but no further. In other words, adding exactly the stoichiometric amount of the second reactant so that there is NO excess of either substance.
Doing this requires several things:
You need to be able to add the second reactant carefully and slowly
You need to be able to measure exactly how much of the second reactant was added
You need a way to know when the reaction is “done”
The first two of these requirements are satisfied together by using a burette (sometimes spelled buret, but never pronounced like beret).
The valve at the bottom allows, when used properly, a solution to be added in tiny amounts when needed and is designed to measure the amount of solution delivered to the 100th of a mL.
The third requirement is satisfied by using an indicator - that is, something that shows, usually by color change, that a reaction is finished.
Titrations are most commonly done with acids and bases and the indicators used change color when the pH of a solution changes beyond a particular value. For instance, phenolphthalien is colorless in an acidic solution, but turns a dark pink in basic solution. Thus, a solution that is barely colored will indicate the end point of the reaction (when neither reactant is in excess).
Let’s imagine that you need to know the concentration of an acid solution (perhaps HCl). You would measure a sample of the acid into a flask and add a small amount of indicator solution. A basic solution (perhaps NaOH), with a known concentration, would be added carefully from a burette until the color change begins and the amount of base added would be measured. The reaction that occurred would be:
HCl + NaOH → NaCl + HOH
and the concentration of the acid could be determined according to the math here. For the sake of the example, we’ll assume that the NaOH solution has a concentration of 0.515 M.
and then






No comments:
Post a Comment