In our discussion of electron orbitals and their energy distribution, we mentioned that the 4s orbital is lower in energy than the 3d orbitals are. That means that when electrons are added to an atom, they fill the 4s before the 3d.
This can interfere with our “reading” of the periodic table. As an example, if we were writing the electron configuration of scandium (#21) it would be logical to write [Ar]4s2 4d1. This, however, is WRONG!
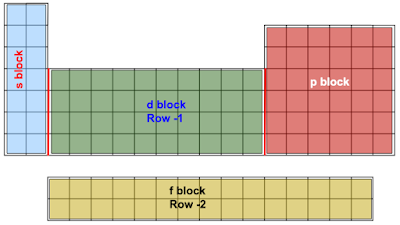
We can, however make a simple adjustment to the periodic table we had before, simply by noting that the d block electrons are on the energy level equal to their row -1. (While we’re at it, it’s worth mentioning that the f block is off by 2.)
So the electron configuration for Scandium is [Ar]4s2 3d1. This pattern continues, so the electron configuration of Rhodium (#45) is [Kr]5s24d7.
The f block is a little more complicated. Not only is the energy level different from the row (as mentioned above) but the f block is actually physically separate from the rest of the table in most cases. That separation is about printing size, NOT about chemistry. The correct construction of the periodic table actually looks like this:
As you can see, the table gets quite wide. In order to print the table in this configuration on a standard piece of paper would require the print to be too small to read.
What matters for our purposes, however, is that the f block appears on the 6th and 7th rows of the periodic table right after the s block. That means that the electron configuration for Promethium (#61) is [Xe]6s2 4f 3, and the electron configuration for Osmium (#76) is [Xe]6s2 4f14 5d8.



No comments:
Post a Comment