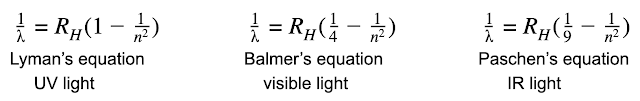Bohr’s theory does an excellent job of explaining the production of light and the Hydrogen spectrum. Of course, nothing is ever easy.
There are only two problems:
a) the math, and
b) the theory
In other words, everything.
Let's look at those two things separately.
The problem with the theory:
Electrons, according to Bohr, jump instantaneously from one ring to another. Understand that Bohr has not claimed that the electrons move from ring to ring. That would require them to be in between levels (an impossible amount of potential energy). Bohr's theory requires electrons to move instantaneously from one ring to another without ever being in between them. This is, of course, physically impossible.
The problem with the math:
Bohr's math predicts the energy and wavelength of the light emitted by a hydrogen atom perfectly. However, the math doesn't work for anything with more than one electron. In other words, it works for Hydrogen, and for a He+1 ion, etc. but doesn't work for an atom of helium.
Since the theory explains the production of light and the math works for hydrogen, we know that there must be some truth to it. However, since the math doesn’t work for MOST things, there must be some real flaws.
It is sometimes easy for students to wonder why we talk about Bohr at all. After all, if he’s wrong, the thought goes, let’s just get to what’s right. So, it’s important to understand that MOST of what Bohr has said IS right.
To be specific:
Energy IS quantized.
Electrons DO occupy energy levels in atoms
Electrons DO generally occupy the lowest energy level they can
When energy is added to an atom, electrons DO move to a higher level
That energy is emitted (as light) when the electron drops back down
So, what’s wrong?
The energy of electrons is not JUST potential energy.
That may seem like a little flaw, but remember it was that idea that created Bohr's picture of rings (or orbits) around the nucleus. If we take away the constraint of potential energy, the picture itself dissolves.
So, the common image of the atom, seen here, is wrong.
So, what do we replace it with? For that we need to go deeper into quantum theory, starting with the photoelectric effect.