Electron Configuration is a short-hand way of describing where electrons are in an atom, that is, which orbitals are occupied by electrons. This idea is incredibly important because the location of electrons is what determines nearly all of the chemical properties and behaviors that we are discussing in this text.
Electron configuration is based on Schrödinger’s theories and mathematical equations and is initially discussed on this page.
Here is a VERY simple summary of what is discussed on those pages:
- Electrons in an atom can be thought of as waves centered around the nucleus
- There are a number of different forms or shapes (called orbitals) that these waves can take
- What orbitals are possible depend on the energy level of the electron
- Electrons are found in the lowest energy orbital possible
- No orbital can hold more than 2 electrons at the same time
- Electrons have a property (called spin) that makes them act like bar magnets
- When more than one orbital has the same energy, electrons will enter these orbitals individually if possible, rather than pairing up.
When we think about how these orbitals are arranged (in terms of energy) it is often easiest to do so visually. We represent the orbitals in a diagram like this:
And we fill the diagram using partial arrows (to indicate spin) like this:
Again, these details can be found here.
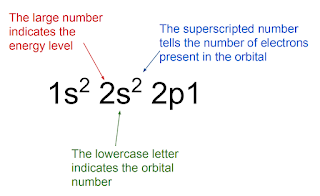
Electron configuration has this format:
It is quite easy to determine electron configuration using a diagram like the one above, however it is not quick and requires one to have access to the diagram (or to recreate it as needed). That's an annoying amount of work, so, naturally chemists don't use these diagrams on a regular basis. They use a shortcut that involves understanding and using the periodic table.
The periodic table was developed BEFORE the discovery of the electron but, because it is based on chemical properties, it aligns with the arrangement of electrons perfectly.
To understand how this works, we'll need to carefully look at the structure of the table.




No comments:
Post a Comment